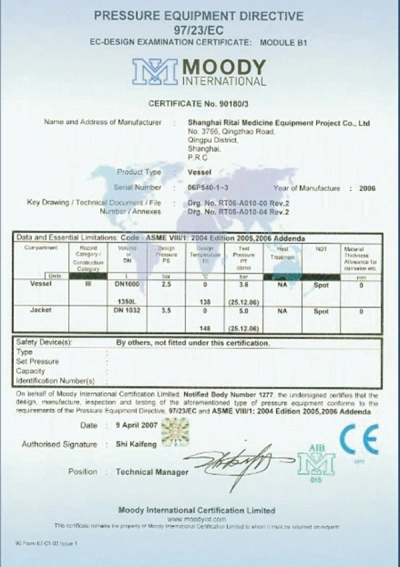
Shanghai Ritai Medicine Equipment Project Co., Ltd. (RITAI) has been dedicated to the R&D of sanitary stainless steel pressure vessels, atmospheric vessels, sanitary valves, and sanitary piping systems, which are extensively utilized in the chemical, pharmaceutical, biological, cosmetic, and food industries since its establishment. RITAI is a proud member of the Chinese Pharmaceutical Equipment Industry Association and the Shanghai Special Equipment Management Association. The company's quality management system is rooted in ISO9001 and holds the manufacturing license for Class I and II pressure vessels in the People's Republic of China, as well as the American ASME (U) stamp.
RITAI possesses advanced technical expertise and state-of-the-art facilities, offering customers comprehensive services encompassing design, manufacturing, installation, commissioning, training, and after-sales support, in compliance with GB, ASME, PED, and other relevant standards and specifications. Our stainless steel vessels and processing equipment have gained popularity not only in China but also in export markets such as the Netherlands, Italy, Jordan, Saudi Arabia, Vietnam, India, Indonesia, Mexico, and Russia.
With a history spanning half a century, RITAI has diligently accumulated extensive expertise in technical and quality management within the realms of design and manufacturing. Our company boasts advanced stainless steel sheet processing, welding, electropolishing, and mechanical polishing technologies. Our products strictly adhere to GMP and FDA requirements, surpassing world-class standards across all quality indicators. Customers consistently praise our products for their exceptional performance, appealing appearance, durability, and outstanding service, contributing to the establishment of our esteemed reputation.
Quality First, Customer First. RITAI has always placed customer satisfaction as a top priority. Leveraging our vast experience and technological prowess, we are committed to continually delivering practical, flawless equipment, alongside high-quality services to our valued customers. As RITAI gradually expands into the international market, our products will become increasingly accessible worldwide.
RITAI animal cell culture R&D center has the capability of cell strain preservation, culture condition screening, high-density culture on microcarriers, fibra disk culture, suspension cell culture and optimization of conditions, as well as bioreactor validation. We can provide customers with services and technical cooperations such as large-scale culture training and guidance, preparation of experimental samples, optimization of tank culture conditions, cultivation scale-up, and rental of experimental equipment, to save costs and time for pharmaceutical development enterprises.