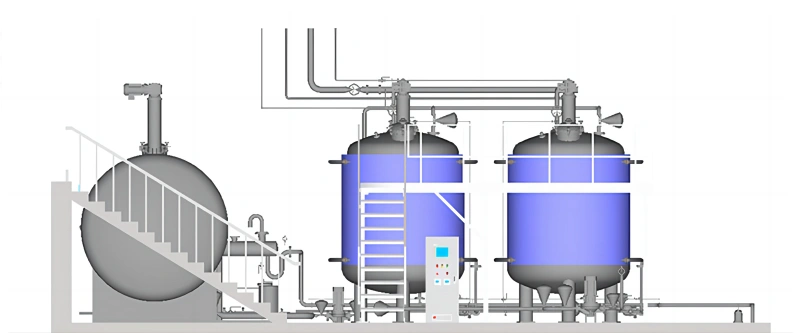
Effluent Decontamination System
Biological waste produced by biopharmaceutical manufacturing typically contains pathogenic microorganisms, such as pathogenic bacteria and viruses, which pose significant hazards to human health. It is necessary for this wastewater to undergo disinfection and sterilization in order to kill the pathogens before being discharged into the sewage treatment system.
Based on the above situation, RITAI has developed a biological wastewater inactivation system specifically designed for physical sterilization methods. This system utilizes sustained and stable high temperatures to denature the bacterial cells or deactivate coagulase, leading to bacterial death. Additionally, viruses experience DNA and RNA chemical absorption of heat at high temperatures, causing bond breakage and subsequent inactivation.
RITAI effluent decontamination system (EDS) utilizes a batch processing approach, incorporating a collection vessel and two or more inactivation vessels. Its intermittent operation ensures energy efficiency and environmental protection during use, while also improving system reliability. Moreover, the entire system is controlled through intelligent automation, enabling unattended and fully automatic operation, and has demonstrated significant results in practical projects.
| Design pressure | 0.4MPa |
| Design temperature | 150℃ |
| Working volume | 1000L, 2000L, 3000L, 5000L to 50000L |
| Sterilization time | 30-120 minutes, adjustable |
| Discharge requirements | Zero living microorganisms |
| Main material (contact surfaces) | 316L stainless steel |
| Material of auxiliary parts | 304 stainless steel |
- Complete inactivation: allows for inactivation sampling verification to guarantee high safety of biological inactivation.
- Intelligent management system: offers unattended operation, and various contingency plans to ensure high reliability of the biological inactivation system.
- Visualize biological sterilization data and progress.
- Heat recovery device: allows recycling.
Dedicated Manufacturer of Bioprocess Equipment Shanghai Ritai specializes in high-end Bioreactors, Fermenters, and Modular Process Systems for the global biopharmaceuticals, enzyme production, and bio-agriculture industries. Beyond premium stainless steel fabrication, we deliver turnkey engineering solutions—from conceptual design and ASME-certified manufacturing to installation and GMP-compliant validation. Trusted by clients worldwide, RITAI ensures your critical equipment meets the strictest international standards, including ASME (U) Stamp, EU PED, and FDA regulations.